When Good Medicines Go Wrong: Rethinking TNF Therapy in Heart Failure
Article Date | 2 September, 2025
By Professor Qamar Javed, Senior Lecturer in Health & Social Science, LSST Luton
Understanding Heart Failure
Chronic heart failure is a progressive condition where the heart gradually weakens and changes shape. Its chambers may enlarge, the walls become stiff or thin, and scar tissue (fibrosis) builds up, reducing the heart’s ability to relax and pump blood efficiently. Muscle cells can die or lose function, their energy production falters, and problems with calcium handling impair contraction. At the same time, stress hormones and inflammatory chemicals remain persistently active, worsening the damage. Together, these changes cause the tiredness, breathlessness, and swelling conditions so often seen in patients.
One key inflammatory player in this process is tumour necrosis factor-alpha (TNF). When blood flow is restricted by narrowed arteries (stenosis), the oxygen-starved heart muscle releases TNF as an “alarm signal.” While intended to help, excess TNF fuels inflammation, promotes scar formation, and accelerates the decline in heart function (Fig 1).
Heart Diagram: Coronary Artery Blockage, Inflammation, and Ischemia
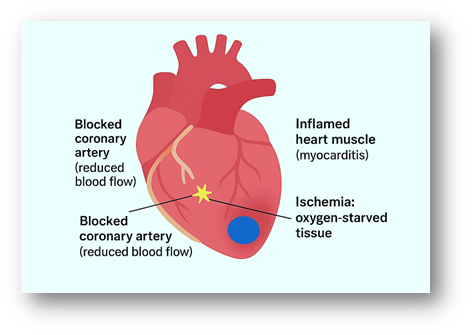
This illustration highlights three major pathological features of heart disease: a blocked coronary artery (yellow), where plaque or clot reduces blood flow; inflamed cardiac muscle (red), representing myocarditis and tissue injury; and ischemic regions (blue), showing oxygen-starved tissue downstream of the blockage. Together, these processes weaken the heart and contribute to the progression of heart failure.
Anti-TNF Therapy: Promise and Setbacks
Because TNF drives inflammation, anti-TNF drugs were developed to block it. In conditions such as rheumatoid arthritis and Crohn’s disease, this approach has worked well. In one of my postdoctoral research projects at the University of Leicester, supported by a personal grant from the British Heart Foundation, I investigated the therapeutic role of anti-TNF therapy in cardiovascular pathology. Our findings showed that local neutralisation of TNF with an anti-TNF antibody reduced vascular hyperplasia (excessive vessel wall thickening) in human vascular tissue, effectively limiting vascular blockage. These results, published in a peer-reviewed journal, highlighted TNF as a promising therapeutic target for cardiovascular disease. Similar strategies from other laboratories, particularly in animal models, have also reinforced the potential of TNF inhibition to mitigate cardiovascular events.
Because heart failure is closely linked to chronic inflammation, doctors hoped that blocking TNF would improve heart health. In inflammatory conditions like rheumatoid arthritis (RA) and Crohn’s disease, anti-TNF treatments have already shown clear benefits, improving patient outcomes. However, the story turned out differently with heart disease. Instead of helping, anti-TNF therapy sometimes made things worse, leading to new cases of heart failure and other cardiac complications.
Clinical trials tested two major drugs: infliximab (INF), designed to neutralise TNF, and etanercept (ETA), which targets TNF receptors. The goal was to lower levels of this inflammatory protein in the blood, thereby reducing heart failure complications. But the results were disappointing; rather than protecting patients, both drugs were linked to worsening cardiac symptoms, higher hospitalisation rates, and in some cases, even an increased risk of death (Fig 2).
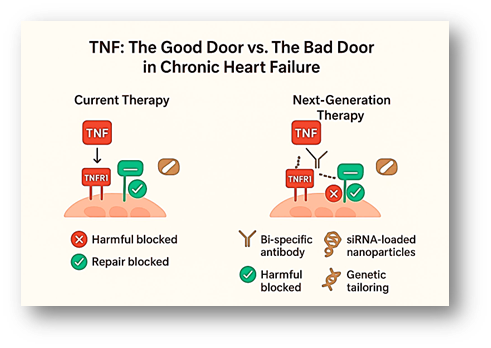
Why Did Treatment Fail?
In my recent mini review published in Molecular Biology Reports, I examined the cellular and molecular mechanisms of TNF signalling within the heart and circulation. TNF interacts with distinct receptors on the surface of cardiomyocytes (heart cells), which can be thought of as “doors” through which its signals pass. TNFR1, often considered the “bad door,” activates inflammatory and cell death pathways, contributing to tissue injury. In contrast, TNFR2, the “good door,” promotes healing and tissue repair in the heart (Fig. 2).
Both INF, and ETA showed disappointing results in the heart failure patients. These disappointing results can be explained by the dual nature of TNF signalling. TNF works through its specific receptors: TNFR1, which drives harmful inflammation, scarring, and cell death of the heart tissue, while TNFR2 promotes cell survival, cardiomyocytes repair, and blood vessel growth.
Anti-TNF therapy drugs (INF, and ETA) blocked both receptors simultaneously. This meant the damaging effects of TNFR1 were reduced, but at the same time, the protective effects of TNFR2 were also lost. In essence, these drugs shut down the heart’s natural repair system while trying to control inflammation (Fig 2).
The New Direction: Receptor-Specific Therapies
Researchers are now developing smarter treatments that work by targeting TNF’s two receptors in different ways. The goal is to switch off TNFR1, which drives harmful inflammation and heart damage, while keeping TNFR2 active, since it supports repair and recovery.
One major innovation is the development of bi-specific monoclonal antibodies (BsAbs). Unlike older TNF inhibitors, BsAbs can do two jobs at once: block the damaging signals from TNFR1 while boosting the protective functions of TNFR2. In heart disease, this means stopping the “bad” signals that fuel inflammation while strengthening the “good” ones that help the heart healing process (Fig. 2). These therapies are already in use for some cancers, and scientists are now testing whether they could also help in heart failure and blood vessel disease.
Early evidence from animal studies and laboratory experiments is promising. BsAbs have been shown to lower inflammation, protect heart cells under stress, and promote the repair of damaged tissue. This approach matters because it moves beyond the old “one-size-fits-all” method of blocking all TNF activity. Instead, receptor-targeted therapies aim for balance, suppressing harmful inflammation without shutting down the heart’s natural healing. If ongoing trials support these findings, receptor-specific therapies could mark the beginning of a new era of safer, more personalised treatments for heart failure.
New ideas are now being tested to make treatment smarter. Another approach is to use tiny carriers called nanoparticles that deliver special molecules (siRNA) to block only the harmful switch, TNFR1. Other nanoparticles can even carry “helper signals” to activate TNFR2, boosting the heart’s natural repair system.
In early animal studies, these targeted treatments reduced scarring, lowered inflammation, and helped the heart pump more effectively. In the future, this kind of precise, personalised therapy could give patients safer and more effective options than the older conventional treatments.
Looking Ahead
The next step for anti-TNF treatment is to match the right therapy to the right patient. Instead of one medicine for everyone, doctors could use simple tests to check a person’s genes and inflammation pattern to see how active the two TNF “switches” are (TNFR1 and TNFR2). New tools make this targeting possible: bi-specific antibodies (BsAbs) can block the harmful TNFR1 signal while supporting the helpful TNFR2 signal, and siRNA medicines can quietly “turn down” TNFR1 inside cells without touching TNFR2. Combined with genetic screening for TNF-related variants, these approaches aim to calm damaging inflammation but keep the natural repair process working, offering a safer, more precise way to protect the heart.
Importantly, growing evidence from genetic studies, including our own published work on TNF gene polymorphisms, shows that individual variability in TNF expression influences disease risk and treatment response. Integrating such polymorphism profiling into therapy design could identify patients who are genetically suited to benefit from anti-TNF approaches, while protecting others from potential harm. By combining receptor-selective strategies with genetic and molecular stratification, future anti-TNF therapies can move beyond a one-size-fits-all approach and advance toward precision-guided immunomodulation in cardiovascular disease.
Closing Note
This article also reflects knowledge from my own research journey. In my postdoctoral projects, I studied how TNF influences blood vessels and contributes to cardiovascular pathology. Later, I supervised PhD students investigating how TNF gene activity shapes the progression of heart failure. By linking these earlier insights with today’s advances, it becomes clear how past research continues to shape the development of new therapies. The hope is clear: by refining anti-TNF strategies to block only the harmful effects while keeping the protective ones, we may offer heart patients safer and more effective care in the future.
It is important to note that current AHA/ESC guidelines do not recommend anti-TNF therapy in heart failure due to associated risks. Accordingly, this review does not advocate their use; rather, it highlights the molecular mechanisms underlying their limited success and explores future strategies. Such strategies include the use of bispecific antibodies (BsAbs), nanomedicine-based delivery systems, and genetic profiling based on TNF polymorphisms to streamline treatment approaches and improve patient outcomes.
This article is a simplified version aimed at students and the general audience; readers who wish to explore the full scientific details and references are encouraged to consult the author’s original publication below:
Published article: Javed, Q. Molecular mechanisms and complications of TNF blockade in heart failure: targeting signalling and genetic determinants of therapeutic response. Molecular Biology Reports 52, 772 (2025). https://doi.org/10.1007/s11033-025-10877-6




